Digitize your GxP reviews
in full compliance
IVTracer is a comprehensive monitoring system that integrates analog and digital sensors, harmonizing environmental and equipment monitoring across cleanrooms and laboratories.
The Paperless module within the IVTracer solution enables fully digital management of critical data reviews in regulated environments. It ensures complete traceability, data integrity protection, and long-term retention of GxP data.
Environmental monitoring data
Digitalization for efficient and responsible management
In an increasingly demanding regulatory environment, manual processing of GxP data reviews is becoming a barrier to efficiency, compliance, and sustainability.
While the digitization of GxP data reviews is not mandatory, the FDA strongly encourages it because it helps to:
✔️ Improve data integrity
✔️ Ensure full traceability
✔️ Comply with FDA 21 CFR Part 11 requirements
That’s why we designed the Paperless module — a digital solution that enables you to digitize your validation, review, and archiving processes, while strengthening data traceability and security.
Zero paper, zero friction, 100% traceable, 100% compliant.
Digitized Reviews
Efficiency and reliability combined
No more paper approvals, multiple signatures, missed steps, or errors. With our Paperless module:
✔️ Accelerate approvals through a streamlined, structured workflow
✔️ Minimize the risk of human error
✔️ Improve the traceability of every action — for easier, faster audits
Every step is recorded, every verification/approval is dated, and every piece of data is secured.
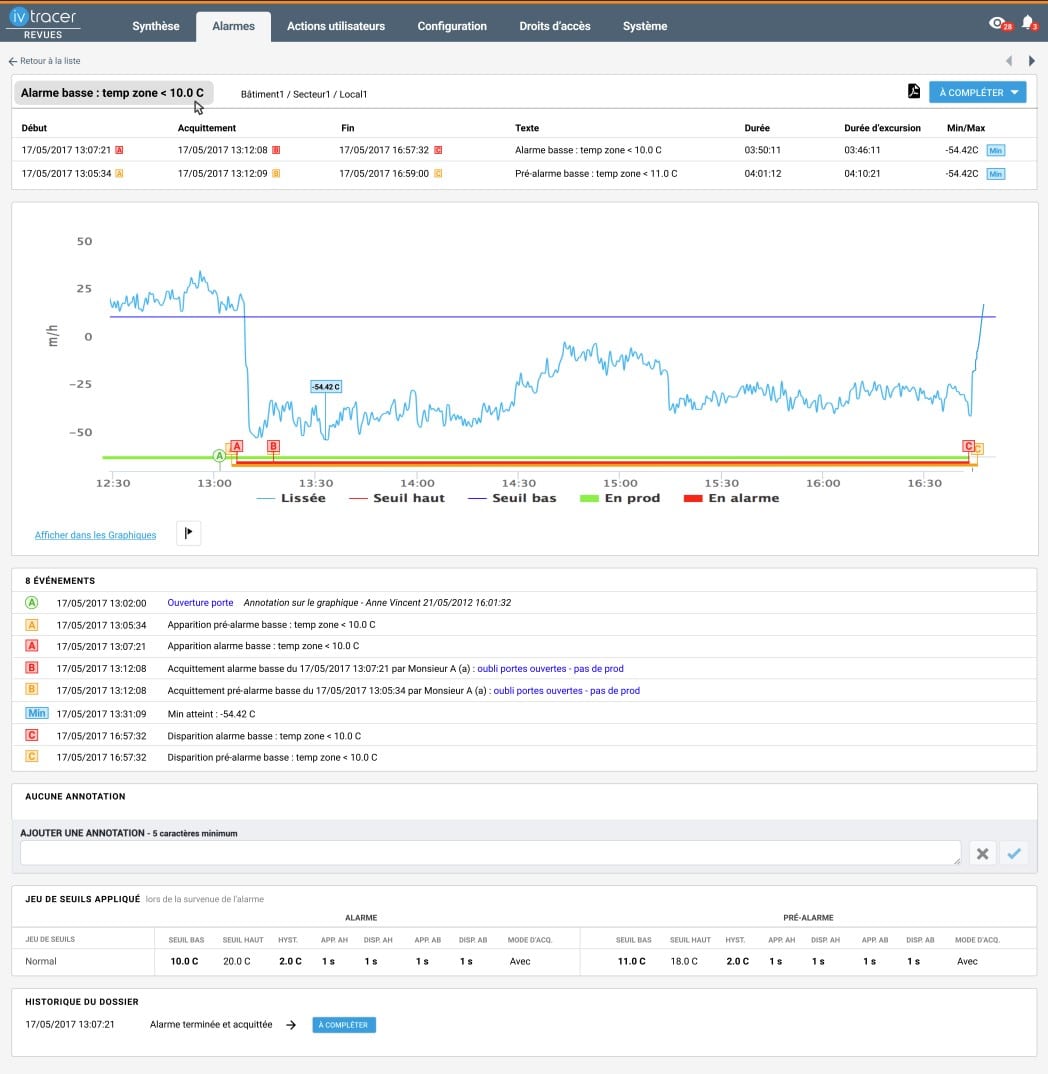
User interface
Simplicity of approval process
The module lets you conduct reviews quickly and access them anywhere through an intuitive interface. No need to be on site to approve an environmental review:
✔️ Review and approve in just a few clicks — on desktop, tablet, or mobile
✔️ Fully automated validation workflows
✔️ Integrated notifications, reminders, and role-based access
✔️ Dedicated workflows for each GxP data type
Your processes become more fluid and your teams more responsive.
security
Secure & auditable archiving
All validations, modifications, and data are automatically archived in a secure environment compliant with GMP, ALCOA+, and Annex 1 requirements:
✔️ Data protected, archived, and traceable at all times
✔️ Centralized access according to user permissions
✔️ Full audit trail for every document
A true digital vault for your critical data.
Environmentally responsible
Zero Paper, Cost Reduction, and Positive Impact
Digitizing your processes makes you faster, more secure — and more environmentally responsible.
✔️ Less printing, less paper to store
✔️ Lower document storage and processing costs
✔️ Reduced environmental footprint
Data management aligned with your CSR and sustainability goals.
With the Paperless module:
〉Fully digitize your GxP data review management
〉Simplify and secure your validation workflows
〉Save time, ensure reliability, and gain peace of mind
〉Contribute to a more sustainable, eco-friendly operation
